
by Svendt | Mar 12, 2016 | Events
. . 2016 PHARMED CRO Forum Contract Research Outsourcing Annual Meeting, Sheraton Laval, April 7, 2016 Super Panel Speakers . François Peaucelle, Executive Director of Biotrial, France Clinical trial incident in France : the Biotrial crisis management, impacts...

by Svendt | Mar 10, 2016 | Events
. . . Press release . . A Memorandum of understanding (MOU) was signed on March 8 between Access Pharma Group and PharMed Business Group, the Health Industries Manufacturing and R&D Outsourcing Network of Canada. The two parties agreed to develop...

by Svendt | Feb 26, 2016 | Events
Notre partenaire BioTech Annecto Montréal organise son événement de réseautage mensuel ce mardi, le 1 mars 2016. Jim Cochrane, SrCRA, Recherche Clinique Cochrane Inc, va parler sur un sujet très intéressant – comment devenir Associé de recherche clinique...

by Svendt | Feb 13, 2016 | Events
Feb. 25th, Toronto, Careers in Clinical Research (Clinical Research Association of Canada), Feb. 25th, Montreal, Soirée Réseautage et Conférences (Montreal University) March 1st, Montreal, Becoming a Clinical Research Associate, (BioTech Annecto-Montreal), April 7th,...
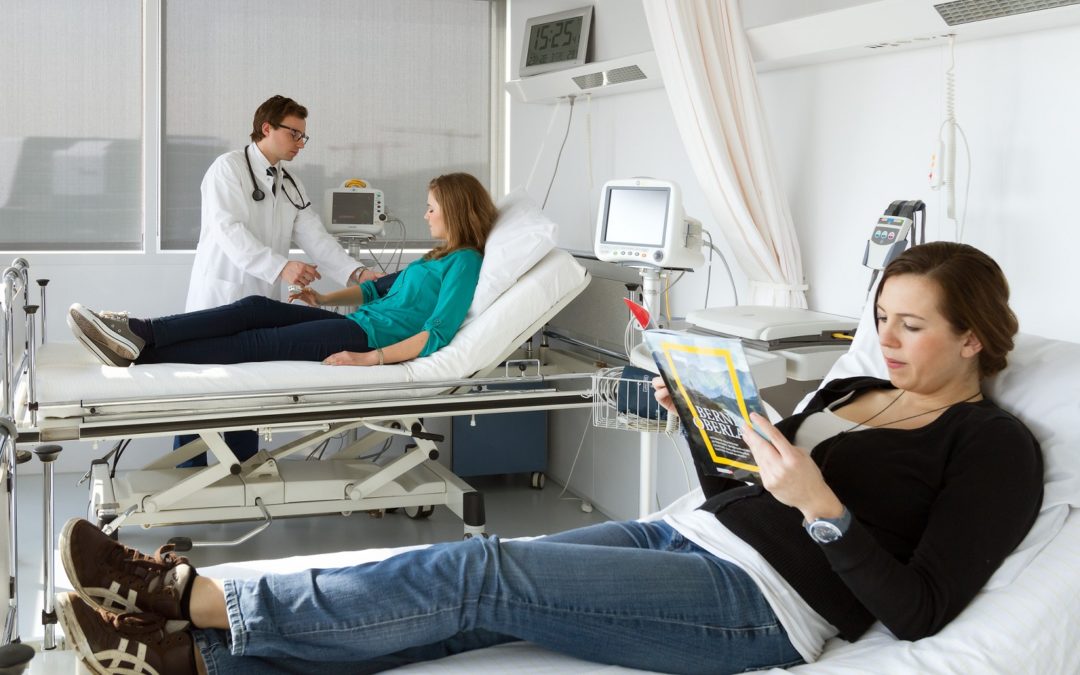
by Svendt | Jan 18, 2016 | Articles
BIA 10-2474 THERAPEUTIC TEST GOES FATAL IN FRANCE. WHAT DID HAPPEN ? WHAT ARE THE REAL RISKS IN PHASE I CLINICAL TRIALS ? WHAT IS A CLINICAL TRIAL? A clinical test normally takes place in 3 phases and the drug development may take in total between 10 and 15...






